Implications of Arctic Sea Ice Reduction on Tropospheric Chemistry
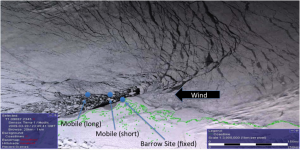
The objective of this interdisciplinary research, anchored by the BRomine, Ozone, and Mercury EXperiment (BROMEX) field study (Figure 1), was to investigate impacts of Arctic sea ice reduction on bromine, ozone, and mercury chemical processes, transport, and distribution, from sea ice surfaces and near leads on the Arctic Ocean, and atmospheric transport of these chemicals to high mountains on land.
Key science questions still remain to be answered in order to understand the impact of the recent drastic reduction of Arctic sea ice, which profoundly changes the Arctic environment, on the physical and chemical processes involved in bromine explosions leading to depletion of tropospheric ozone (O3) and gaseous elementary mercury (GEM), specifically:
- Will the Arctic sea ice reduction, which has shifted the state of the Arctic sea ice cover to the dominant domain of seasonal ice, continue the declining trend?
- How is the chemical process for bromine explosion initiated from the surface with different sea ice classes (seasonal and perennial ice), frost flowers, snow cover, and open ocean that are affected by the Arctic sea ice change?
- How is bromine recycled and distributed in the tropospheric vertical column and how are bromine explosion events propagated and terminated? What are the relative roles of vertical mixing/dilution versus depositional loss of bromine products to a relatively non-saline surface?
- How are bromine and thus O3, GEM, and reactive gaseous mercury (RGM) distributed in time (seasonal, interannual, and decadal) and in space (on ice/ocean, near shore, inland, and around mountain complex) and what is their relationship to oceanic and atmospheric forcing in the changing Arctic environment?
- If the Arctic sea ice reduction trend continues, how would the physical and chemical conditions above the surface change, and would the frequency and severity of bromine explosions and O3/GEM depletions increase or decrease?
Answers to the above key questions provide fundamental physical insights into the halogen chemical processes, which govern bromine, ozone, and mercury in the Arctic environment that is impacted by a changing Arctic sea ice surface and related changes in climate. Such understanding is critical to correctly identify the cause and consequence in Arctic O3/GEM depletion in response to Arctic change, and enables a physical-based assessment of the Arctic environmental vulnerability to geochemical change. Scientifically, a fundamental understanding is essential to enable the research community to develop reliable Earth System and Arctic System Models that connect the physical changes at the surface to the chemical compositional changes in the atmosphere, with attendant feedbacks, so that we can address questions about the future state of the Arctic system. In a practical view, tropospheric (the air the we breath) ozone and mercury are toxic to people and wildlife. Understanding the chemical sources processes, and transport will enable a better quantification of the impacts.
Our approach used data from multiple satellites including MODIS (NASA), AMSR-E (NASA), QuikSCAT (NASA), Envisat ASAR (ESA), GOME-2 (ESA), SCIAMACHY (ESA), RADARSAT SAR (CSA), and TerraSAR-X and TanDEM-X (Germany) together with past and present measurements from multiple field campaigns such as the IPY OASIS, INCATPA, CFL, SALT, and other present and future field experiments, to investigate how changing sea ice conditions can change the rates and nature of chemical processes involving these species. The approach included a new unique field experiment to identify the role of different sea ice surface types in the photochemical processes, together with airborne measurements across various Arctic land-seascapes. We planned to conduct measurements from both sides of the Barrow lead in the spring of 2012, from the surface (using autonomous platforms) and from aircraft, making use of trajectory analysis and satellite data, along with the chemical information, to study the relationship between surface types and the overlying atmospheric composition and chemistry. We used the Drift-age Model to characterize sea ice dynamics and distribution. Furthermore, atmospheric dynamics were included in this research using model analyses such as the National Centers for Environment Prediction and National Center for Atmospheric Research reanalysis and Rising Air Parcel (RAP) trajectories.
Based on new findings, we assess the capability for chemical weather forecasting in the Arctic, specifically aimed at short-term prediction of bromine explosions and O3/GEM depletion. Thus, this research built on and expanded NASA multi-satellite capabilities leading to the first assessment and potential development of new predictive modeling for chemical weather forecast. Thus, it lent support to Decadal-Survey satellite missions related to ozone and ice (e.g., CLARREO, GPSRO, ASCENDS, GACM, GEO-SCAPE, ACE, XOVWM, DESDynI, ICESAT-II, etc.).
Investigators:
- Son V. Nghiem, NASA/JPL, Principal Investigator
- Matthew Sturm, Cold Regions Research and Engineering Laboratory
- Thomas Douglas, Cold Regions Research and Engineering Laboratory
- Don Perovich, Cold Regions Research and Engineering Laboratory
- Paul Shepson, Purdue University
- William Simpson, University of Alaska
- Ignatius Rigor, University of Washington
Collaborators:
- David G. Barber, University of Manitoba, Canada
- Jan Bottenheim, Environment Canada
- John P. Burrows, Oxfordshire, United Kingdom; and University of Bremen, Germany
- Pablo Clemente-Colón, National Ice Center, U.S.
- Hajo Eicken, University of Alaska
- CDR Carl Hager, CDR Joe Smith, LCDR John Woods, U.S. Naval Academy
- Dorothy K. Hall, NASA Goddard Space Flight Center, U.S.
- Lars Kaleschke, University of Hamburg, Germany
- Jeremy Krieger, University of Alaska, Fairbanks
- Thorsten Markus, NASA Goddard Space Flight Center, U.S.
- Andreas Richter, University of Bremen, Germany
- Ralf Staebler, Environment Canada
- Alexandra Steffen, Environment Canada
For more information, contact Son V. Nghiem, Jet Propulsion Laboratory
